Designing Oxides via Low Temperature Synthesis
Generally, the designed synthesis of oxides is difficult under high temperatures. We plan to circumvent this problem by developing a toolbox of low-temperature reactions instead.
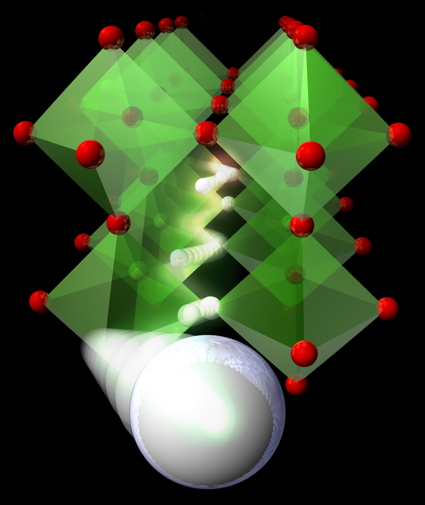
The reduction of the simple compound SrFeO3 shown on the right illustrates one of our approaches (2007). In the movie on the right, oxygen anions (red spheres) are removed in an orderly manner from SrFeO3 to form SrFeO2, resulting in a structure composed of square planar FeO4 units. This is highly unusual, given that iron is generally known to adopt only tetrahedral or octahedral coordination. The fact that such a novel geometry comes from such a simple method and composition is a testament to the wide range of possibilities of these low-temperature synthesis techniques.
Hydrogen moves! Seemingly bouncing around, hydride (H-; white spheres) can exist quite comfortably in simple oxides such as BaTiO3, and furthermore diffuse throughout the lattice.