see recent publications (at the bottom of this page)
Organic molecules have various physical and biological properties derived from their structures, and therefore the construction of such molecules by reasonable and efficient ways has been an important issue in organic synthesis. Our research group explores novel organic reactions and novel molecular properties by employing organic active species such as cations, radicals, anions, carbenes, etc. as key components. We also study the biological properties of the synthesized functionalized molecules as bioimaging probes in vitro as well as contrast agents in vivo.
Introduction of our research in 2023 (pdf, in Japanese)

【Current research topics】
- Transition metal-catalyzed reactions for construction of heteroaromatics
Heteroaromatics are attractive organic compounds and applied as biological as well as electronic materials. We are exploring new and efficient transition metal-catalyzed reactions to construct heteroaromatics from accessible starting compounds.
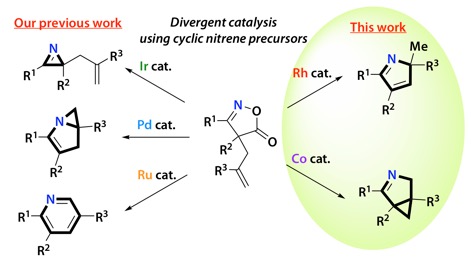
Divergent Catalytic Approach from Cyclic Oxime Esters to Nitrogen-Containing Heterocycles with Group 9 Metal Catalysts
Takuya Shimbayashi, Gaku Matsushita, Atsushi Nanya, Akira Eguchi, Kazuhiro Okamoto,* and Kouichi Ohe*
ACS Cat., 8, 7773-7780 (2018).doi.org/10.1021/acscatal.8b01646
- Stimuli-responsive molecular probes for evaluation of biological process
We are designing and synthesizing new types of stimuli-responsive probes for detection of biological processes, especially enzyme and physiological conditions (pH). We are currently exploring dual-responsive molecular probes for detection of cancer stem cells and so on.
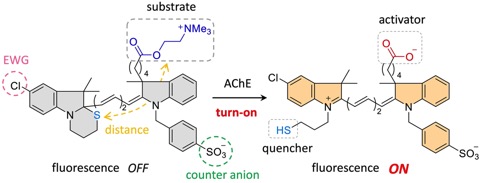
An activator-induced quencher-detachment-based turn-on probe with a cationic substrate moiety for acetylcholinesterase
Masahiro Oe, Koji Miki*, Akito Masuda, Kohei Nogita and Kouichi Ohe*
Chem. Commun. 2022, 58, 1510-1513. DOI: 10.1039/d1cc05132f
Selected as a front cover

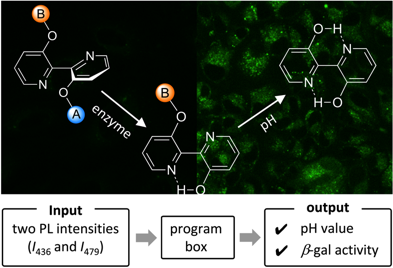
Dual-Stimuli-Responsive Probes for Detection of Ovarian Cancer Cells and Quantification of Both pH and Enzyme Activity
Wenting Huo, Koji Miki*, Daisuke Tokunaga, Huiying Mu, Masahiro Oe, Hiroshi Harada, Kouichi Ohe*
Bull. Chem. Soc. Jpn. 2021, 94, 2068-2075. DOI: 10.1246/bcsj.20210168
[Selected Paper]

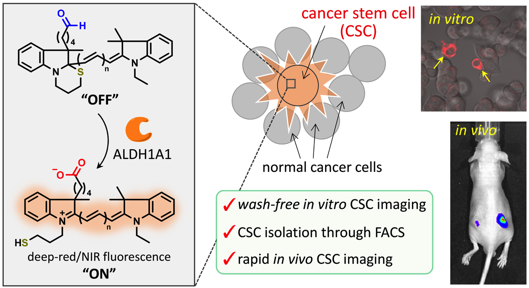
Deep-Red/Near-Infrared Turn-On Fluorescence Probes for Aldehyde Dehydrogenase 1A1 in Cancer Stem Cells
Masahiro Oe, Koji Miki*, Yoshifumi Ueda, Yasuo Mori, Aoi Okamoto, Yohei Funakoshi, Hironobu Minami, and Kouichi Ohe*
ACS Sens. 2021, 6, 3320-3329. DOI: 10.1021/acssensors.1c01136
Substituted meso-Vinyl-BODIPY as Thiol-Selective Fluorogenic Probes for Sensing Unfolded Proteins in the Endoplasmic Reticulum
Huiying Mu, Koji Miki,* Takuya Kubo, Koji Otsuka,
and Kouichi Ohe*
Chem. Commun., in press. DOI: 10.1039/D0CC08160D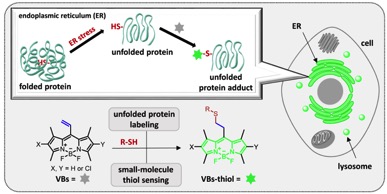
- Tumor imaging contrast agents in vivo
We are focusing on the synthesis of contrast agents for optical and photoacoustic tumor imaging and tomography. By applying stimuli-responsive probes, we recently reported the activatable photoacoustic contrast agent for detection of acidic environment in tumors.
Reductively convertible nickel phthalocyanine precursor as a biological thiol-responsive turn-on photoacoustic contrast agent
Kohei Nogita, Takaya Sugahara, Koji Miki,* Huiying Mu, Minoru Kobayashi, Hiroshi Harada, and Kouichi Ohe*
Chem. Commun. 2024, accepted.
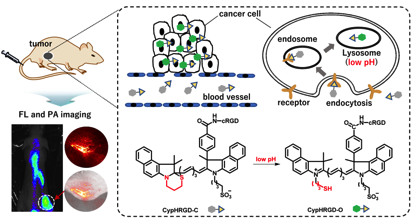
pH-Activatable Cyanine Dyes for Selective Tumor Imaging Using Near-infrared Fluorescence and Photoacoustic Modalities
Huiying Mu, Koji Miki,* Hiroshi Harada, Kouki Tanaka,
Kohei Nogita, and Kouichi Ohe*
ACS Sens., 2021, 6, 123-129. doi.org/10.1021/acssensors.0c01926