Research targets
Overview
Depletion of fossil resources and other environmental issues have become a matter of serious concern, and researchers are now expected more strongly than ever to contribute to the realization of sustainable development - a society that balances the economy with the natural environment. It has been estimated that the amount of available solar energy on the surface of the Earth is much higher than the total energy consumption by humankind. Therefore, the development of an efficient solar-light energy conversion system could be of tremendous help in meeting our future energy need. Our research group is aiming at development of highly efficient photocatalytic reactions over semiconductor materials for solar energy conversion and environmental purification. Main research targets are:
(1) New photocatalytic water splitting systems toward efficient solar hydrogen production
(2) Highly efficient visible-light-responsive photocatalysts for environmental purification
(3) Highly selective photocatalytic reactions for organic synthesis
Subject of Research
(1) Development of new photocatalytic water splitting systems toward efficient solar hydrogen production
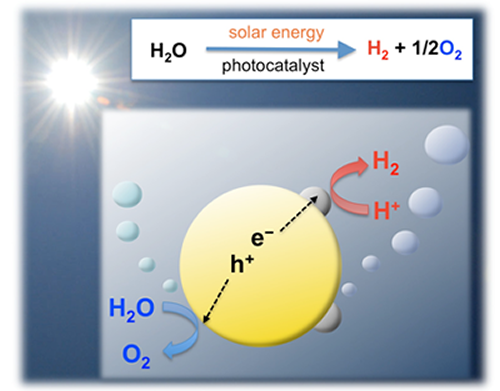
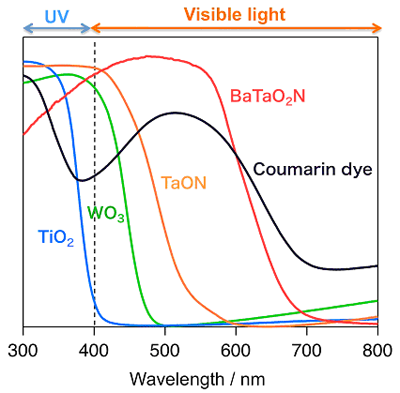
The development of a clean and renewable energy carrier that does not utilize fossil fuels is a great technological challenge. One of the most attractive options is the large-scale utilization of hydrogen (H2) as a recyclable energy carrier. However, industrial H2 production consumes huge amounts of fossil fuels (e.g., natural gas), resulting in equally large CO2 emissions. Photocatalytic water splitting using semiconductor materials (see Fig. 1) has thus attracted considerable interest due to its potential for clean production of H2 from water by utilizing abundant solar light. Ever since Fujishima and Honda reported photoelectrochemical water splitting using a TiO2 electrode in 1972, numerous researchers have intensively studied water splitting using semiconductor photoelectrodes or photocatalysts. Although more than 100 photocatalytic systems based on metal oxides have been reported to be active for “overall” water splitting (i.e., simultaneous generation of both H2 and O2), most of them function only under ultraviolet (UV) light (λ< 400 nm) because of the large band-gap energy of semiconductor materials. Since nearly half of the solar energy incident on the Earth’s surface lies in the visible region (400 < λ < 800 nm) (see Fig. 4), it is essential to use visible light efficiently to realize practical H2 production on a huge scale by photocatalytic water splitting.
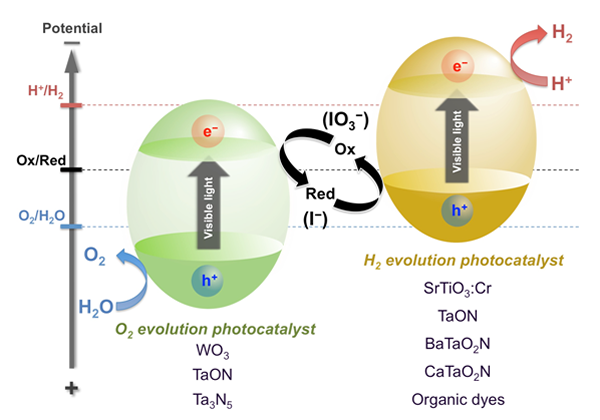
We have developed a new type of photocatalysis system that can split water into H2 and O2 under visible light irradiation (see Fig. 2), which was inspired by the two-step photoexcitation (Z-scheme) mechanism of natural photosynthesis in green plants. In this system, the water splitting reaction is broken up into two stages: one for H2 evolution and the other for O2 evolution; these are combined by using a shuttle redox couple (Red/Ox) in the solution. We have achieved overall water splitting using various visible light responsive photocatalysts, such as SrTiO3 co-doped with Cr and Ta (SrTiO3:Cr/Ta), tantalum oxynitrides (TaON or BaTaO2N), and coumarin organic dyes, which work as a H2 evolution photocatalyst, combined with tungsten oxide (WO3) for O2 evolution in the presence of a shuttle redox mediator such as iodate/iodide (IO3-/I-). For example, the combination of Pt-SrTiO3:Cr/Ta and Pt-WO3 photocatalysts resulted in simultaneous evolution of H2 and O2 from an aqueous NaI solution under visible light irradiation ( λ > 410 nm), as shown in Fig. 3. This was the first demonstration of water splitting into H2 and O2 under visible light irradiation. It represents one of the most important technical breakthroughs in the field of photocatalytic water splitting. The use of BaTaO2N or coumarin organic dye was demonstrating to be photoactive at wavelength up to ca. 700 nm. These results demonstrate the potential of a two-step water-splitting system for utilizing a broader band of visible spectrum (see Fig. 4). Another advantage of Z-scheme systems is the ability to separate production of H2 and O2 by employing a separator, such as a porous glass filter, that permits only redox mediators to be transferred. In the practical application, the separation of H2 from O2 is quite important to avoid explosion of the stored mixture of H2 and O2.
Our recent publications
ChemSusChem (Invited paper), 4, 228-237 (2011).
Langmuir, 26, 9161-9165 (2010).
J. Am. Chem. Soc., 132, 5858-5868 (2010).
Chem. Commun., 2009, 3577-3579 (2009).
Chem. Mater., 21, 1543-1549 (2009).
J. Phys. Chem. B, 109, 16052-16061 (2005).
Chem. Commun., 2005, 3829-3831 (2005).
J. Photochem. Photobiol. A: Chem., 166, 115-122 (2004).
Chem. Phys. Lett., 371, 360-264 (2003).
Chem. Commun., 2001, 2416-2417 (2001).
Chem. Phys. Lett., 344, 339-344 (2001).
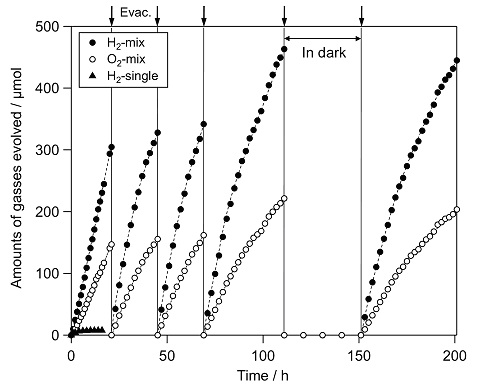 Fig. 3 Time course of photocatalytic evolution of H2 and O2 using a mixture of Pt(0.3 wt%)/SrTiO3 (Cr, Ta 4 mol% doped) and Pt(0.5 wt%)/WO3 photocatalysts suspended in 5 mM of NaI aqueous solution (pH 6.5 without
adjustment) under visible light irradiation ( λ > 420 nm). Triangles
indicate H2 evolution using Pt/SrTiO3:Cr/Ta alone.
Fig. 3 Time course of photocatalytic evolution of H2 and O2 using a mixture of Pt(0.3 wt%)/SrTiO3 (Cr, Ta 4 mol% doped) and Pt(0.5 wt%)/WO3 photocatalysts suspended in 5 mM of NaI aqueous solution (pH 6.5 without
adjustment) under visible light irradiation ( λ > 420 nm). Triangles
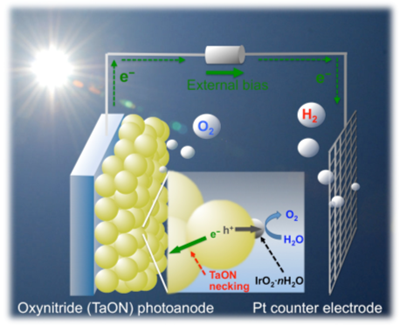
indicate H2 evolution using Pt/SrTiO3:Cr/Ta alone.Since the report of photoelectrochemical (PEC) water splitting using TiO2 electrodes under UV light, extensive efforts have been made to construct an efficient PEC water-splitting system and to develop new semiconductor materials that can utilize visible light for efficient photoelectrodes. We have reported several oxynitrides of transition metals as potential candidates for visible light induced water splitting, since they possess appropriate band levels for both water oxidation and reduction and a narrow band gap that allows visible light absorption. We have recently developed a facile fabrication method for an efficient (oxy)nitride (e.g., TaON) photoanode, in which (oxy)nitride semiconductor particles are first deposited on a conductive substrate using electrophoretic deposition, and then a necking treatment is applied to form effective contacts between the (oxy)nitride particles. We have demonstrated that the porous oxynitride TaON film electrode prepared on conducting glass (FTO) showed significantly high quantum efficiency (IPCE = ca. 76% at 400 nm at 0.6 V vs. Ag/AgCl) for water oxidation, after loading of IrOx nanoparticles as a cocatalyst for water oxidation (see Fig. 5). Overall water splitting into H2 and O2 under visible light was indeed demonstrated using an IrOx-loaded TaON (or Ta3N5) photoanode combined with a Pt electrode under an externally applied bias (TaON: >0.6 V, Ta3N5 > 1 V), which are much lower values compared to those required in previous reported systems.
Our recent publications
Energy Environ. Sci., 4, 4138-4147 (2011).
J. Am. Chem. Soc., 133, 12334-12337 (2011).
J. Am. Chem. Soc., 132, 11828-11829 (2010).
(2) Development of highly efficient visible-light-responsive photocatalysts for environmental purification
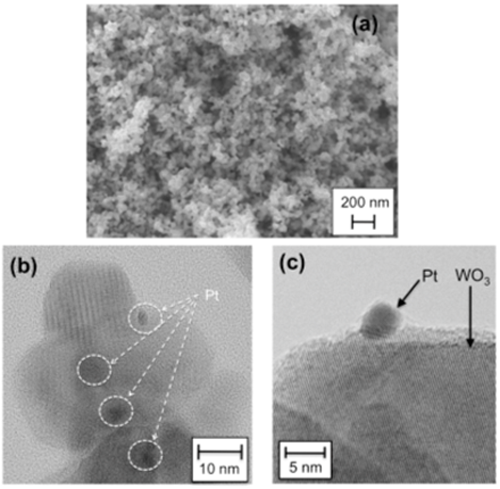
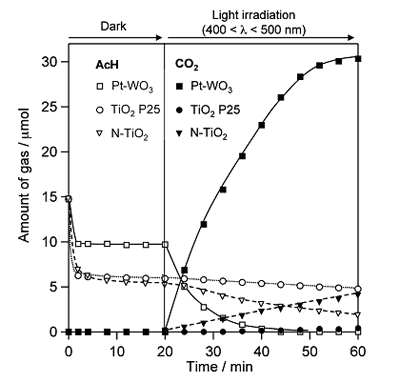
The development of visible-light responsive photocatalysts for environmental purification has been an active research field in recent years, with most research focused on achieving the efficient decomposition of environmental organic contaminants under sunlight or indoor light. Tungsten oxide (WO3) has so far been regarded as inactive photocatalyst for the oxidative decomposition of organic compounds in air, due to the much lower level of CB (ca. +0.5 V) compared to the O2 reduction. Recently, we have reported that crystalline WO3 loaded with nanoparticulate platinum (see Fig. 6) exhibits significantly high photocatalytic activity for the decomposition of various organic compounds under visible light. Figure 7 shows the change in the amount of acetaldehyde (AcH) and CO2 in the gas phase during reaction over Pt(0.1 wt%)-loaded WO3, a commercial titanium oxide (TiO2, P25), and a nitrogen-doped TiO2 (N-TiO2) under visible light irradiation ( λ > 400 nm). With the onset of visible light irradiation, the amount of AcH in gas phase over Pt-WO3 decreased rapidly accompanied by an increase in CO2 generation. Stable CO2 generation was observed over the conventional visible light-responsive photocatalyst N-TiO2, although at a rate much lower than that over Pt-WO3. We found that the high activity of Pt-WO3 is due to the promotion of mutilielectron reduction of O2 on the Pt rather than single-electron reduction, which is generally considered as the main pathway for electron consumption over TiO2 photocatalysts.
Based on these findings, we are currently working on a collaborative project with Sumitomo Chemical Co. Ltd, for the commercialization of WO3 photocatalysts. We have already succeeded in the preparation of aqueous dispersion of the WO3 photocatalyst, which can easily form a transparent film without calcination, and show high activity for the decomposition of various organic compounds under visible light irradiation. The WO3-based photocatalyst, which was named “iLUMiO”, showed significantly high efficiency for the decomposition of smells (cigarette, feces and urine), resulting in imperceptible levels even under indoor fluorescent light irradiation. We have also confirmed that our photocatalyst “iLUMiO” exhibited outstanding activities for both antI-fogging effect and bactericidal effect even under weak fluorescent light.
Our recent publications
Chem. Lett., 40, 443-445 (2011).
J. Mater. Chem., 20, 1811-1818 (2010).
Chem. Commun., 2008, 6552-6554 (2008).
J. Am. Chem. Soc., 130, 7780-7781 (2008).
(3) Development of highly selective photocatalytic reactions for organic synthesis
One of the most attractive targets of semiconductor photocatalytic reaction is its practical application to organic syntheses, as well as solar-energy conversion and mineralization and/or detoxification of organic compounds. Photocatalysis on semiconductor materials may be one of the candidates for environmentally benign synthesis of useful organic compounds, because most of photocatalytic reactions can proceed efficiently even in aqueous suspensions at ambient temperature, by utilizing the energy of irradiated light (natural sunlight or artificial light).
For example, phenol is the major source of phenol resins, which are utilized in many commodities throughout the world. However, its industrial production still requires multistep reaction processes, namely cumene method, which consumes considerably large energy and yields a by-product, acetone. Direct synthesis of phenol from benzene in a one-step reaction, especially using environmentally benign oxidants such as molecular oxygen (O2) or water, is highly desirable, and thus have been extensively studied. We have recently demonstrated for the first time that tungsten(VI) oxide loaded with nanoparticulate platinum (Pt/WO3) to exhibit photocatalytic activity for direct synthesis of phenol from benzene using water and molecular oxygen as reactants under ultraviolet or visible light irradiation. The selectivity for phenol (e.g., 74% at 69% of benzene conversion) on Pt/WO3 photocatalysts was much higher than those on platinum-loaded titanium(IV) oxide (Pt/TiO2) photocatalysts.
We have also proposed a new concept of energy-cycling, in which a fuel-oxidation process produces clean electricity through an efficient alkaline-type fuel cell without CO2 release, and the oxidative products are regenerated to provide the original fuel by an efficient photocatalytic reduction processes that utilizes solar energy. For this achievement, we are now attempting to develop efficient photocatalytic or photoelectrochemical systems that allow the oxidative products (e.g., carboxylic acids, nitrates, and nitrogen) to be reduced to the original fuels (e.g., alcohols and ammonia) under light irradiation.
Recent our publications
Chem. Lett., 40, 1405-1407 (2011).
J. Am. Chem. Soc., 133, 1150-1152 (2011).